Assessment of bone mineral density in multi-transfused thalassemia patients in a tertiary care hospital
Shah S.1, Shah J.2*, R. Vyas B.3
DOI: https://doi.org/10.17511/ijpr.2020.i01.06
1 Sonal Shah, Additional Professor, Department of Pediatrics, G.G.G. Hospital, Jamnagar, Gujarat, India.
2* Jil N. Shah, Senior Registrar, Department of Pediatrics, Gmers Medical College and Civil Hospital, Ahmedabad, Gujrat, India.
3 Bhadresh R. Vyas, Professor, Department of Pediatrics, G.G.G. Hospital, Jamnagar, Gujrat, India.
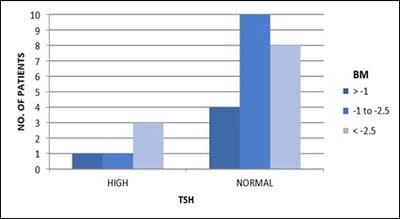
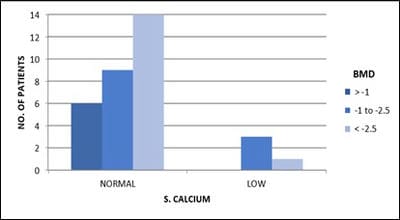
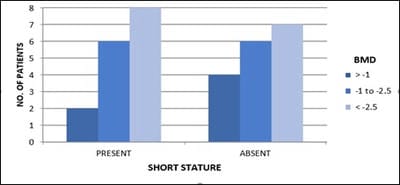
Objective: Patients with transfusion-dependent thalassemia (TDT) are susceptible to osteopenia and osteoporosis, the mechanism is multi-factorial. Transfusion-related iron overload in endocrine organs leads to impaired hormonal secretion, all contributing to bone loss. The aim is the assessment of bone loss frequency and related parameters affecting it in TDT patients in tertiary care Centre, Jamnagar. Design: Observational cross-sectional study. Subjects: 33. Methods: The study is conducted over 1 year in TDT patients attending G.G.G. hospital for blood transfusion. After clearance from the ethical committee and consent from parents, patients selected according to inclusion criteria and scanned for Bone Mineral Density (BMD) and biochemical tests. Results analyzed with the help of standard statistical tests. Results: Out of 33 patients, 36% (12) patients had osteopenia while 45% (15) patients had osteoporosis. 84% (16) of 19 male patients and 78% (11) of 14 female patients had low BMD. 80% (4) of 5 patients with high S.TSH levels and all patients with low calcium levels, were detected with low BMD.11% (3) of 27 patients having low BMD, had high S. Alkaline phosphatase level. 85% (6) of 7 patients with low S.FSH levels and 73% (8) of 11 patients with low S.L.H. levels have low BMD. 87% (14) of 16 patients with short stature have low bone mass. Conclusion: Evaluating BMD annually in TDT patients helps prevent and intervene timely. Administration of bisphosphonates, calcium, vitamin-D supplements, hormone replacement therapy, bone-forming agents and newer therapies will help improve bone mass. Changing lifestyle with appropriate diet and exercise, iron chelation therapy, early diagnosis and treatment of endocrine insufficiency and regular blood transfusions help achieve optimal bone density.
Keywords: Bone loss, Bone mineral density, Osteoporosis, Thalassemia
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Registrar, Department of Pediatrics, Gmers Medical College and Civil Hospital, Ahmedabad, Gujrat, India. Email: |
Shah S, Shah JN, Vyas BR. Assessment of bone mineral density in multi-transfused thalassemia patients in a tertiary care hospital. Pediatric Rev Int J Pediatr Res. 2020;7(1):32-38. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/559 |


 ©
©