Clostridium tetani infection in pediatric intensive care unit of the CHU of Tamatave Madagascar
Aimé Ratsimbazafy A.1, Maharo Andrianarivelo A.2*, Rakotomahefa M.3, Toavinjo Rakotoarivony S.4, Rakotoarisoa H.5, Croix Rasolonjatovo J.6
DOI: https://doi.org/10.17511/ijpr.2020.i02.07
1 Arthur Bien Aimé Ratsimbazafy, Pediatrics Department, Faculty of Medicine Tamatave, Madagascar.
2* Andry Maharo Andrianarivelo, Microbiology Laboratory of the Joseph Ravoahangy Andrianavalona University Hospital Center, Faculty of medicine Antananarivo, Madagascar.
3 Mbola Rakotomahefa, Pediatrics Department, Faculty of Medicine Tamatave, Madagascar.
4 Soloarivelo Toavinjo Rakotoarivony, Multipurpose Intensive Care Unit, Faculty of Medicine Tamatave, Madagascar.
5 Heriniaina Rakotoarisoa, Pediatrics Department, Faculty of Medicine Tamatave, Madagascar.
6 Jean De La Croix Rasolonjatovo, Pediatrics Department, Faculty of Medicine Tamatave, Madagascar.
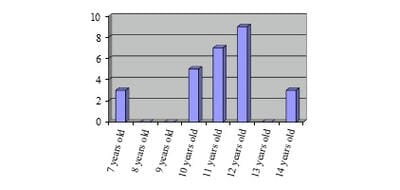
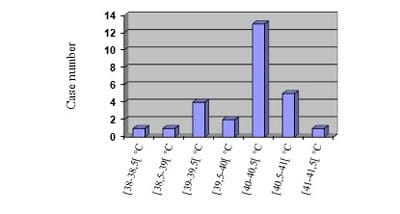
Justification: Tetanus, fatal disease, still exists in Madagascar in spite of the vaccination sessions of mass, and attacks also the children. Objective: To describe the epidemiology, the clinical aspect, the treatments and the evolutions of infantile tetanus. Patients and methods: Retrospective study in the pediatric intensive care unit of the University Hospital of Tamatave, on the cases listed during four years (2013-2016). Results: Twenty-seven cases were recensed, with an average age of 10.8 years old and a sex ratio (M/W) equal to 6. Only forty-one percent were already vaccinated before the one year age. No child of more than 11 years-old profited from vaccine recall. The stain of the wound after the ablation of flea was the most frequent cause (76%). Tetanus was generalized in 80%, including 44.4% with respiratory disorder having required intubation. All the children presented hyperthermia above 38°C at the entry and 63% higher or equal to 40°C. Sedation by diazepam, used at all children, was used with an average amount of 4 mg/kg/day. The beta-lactamine antibiotics were used in 100% of the cases. The antitetanus serum was administered at 3000 IU/day. Nosocomial infections occurred in 61%. Discussion and conclusion: The vaccine recalls are still negligent in the old children, making these latter vulnerable. Mortality is especially due to superinfections and denutrition.
Keywords: Tetanus, Paediatric, Tamatave, Resuscitation
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Microbiology Laboratory of the Joseph Ravoahangy Andrianavalona University Hospital Center, Faculty of medicine Antananarivo, , , Madagascar. Email: |
Ratsimbazafy ABA, Andrianarivelo AM, Rakotomahefa M, Rakotoarivony ST, Rakotoarisoa H, Rasolonjatovo JC. Clostridium tetani infection in pediatric intensive care unit of the CHU of Tamatave Madagascar. Pediatric Rev Int J Pediatr Res. 2020;7(2):87-92. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/564 |


 ©
©