Role of serum procalcitonin in monitoring the response to treatment of pediatric meningitis
Ahmad M.1, Iqbal J.2, Ahmad Wani F.3, Wajid Ali S.4
DOI: https://doi.org/10.17511/ijpr.2020.i07.06
1 Mudasir Ahmad, DNB Scholar, Department of Pediatric Cardiology, Fortis Escorts Heart Institute, New Delhi, , India.
2 Javeed Iqbal, Associate Professor, Department of Neonatology and Pediatrics, Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, Jammu and Kashmir, India.
3 Feroz Ahmad Wani, Lecturer, Department of Community Medicine, Govt. Medical College, Srinagar, Jammu and Kashmir, India.
4 Syed Wajid Ali, Ex-Professor, Department of Neonatology and Pediatrics, Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, Jammu and Kashmir, India.
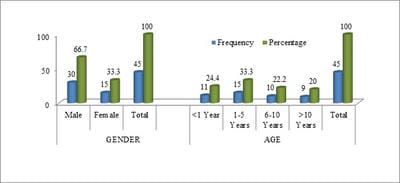
Introduction: A good early diagnostic and prognostic marker for bacterial meningitis will decrease the morbidity and mortality due to this infection. Serum procalcitonin has been evaluated for usefulness in diagnosis and as a prognostic marker in bacterial meningitis. Methods: Children from 5 months to 15 years of age who were cases of bacterial meningitis as per WHO Criteria and were admitted to the Pediatric Department in SKIMS Srinagar, Jammu, and Kashmir were taken for the study. A total of 45 bacterial meningitis cases participated in this prospective study. Serum PCT was measured by a fluorescence immunoassay using QDX Insta check with a detection limit of 0.25-100 ng/ml. Data were analyzed using standard statistical tests using SPSS 20. Results: The mean serum PCT on admission in ng/ml in the present study for bacterial meningitis was 14.9293±4.49122 and after 72 hours mean serum PCT was 12.4386±4.40964). A significant drop (mean difference = 2.49086; p=0.000) in serum procalcitonin was seen after 72 hours following antibacterial treatment. Conclusion: It was concluded that serum PCT can be used as a good prognostic marker to see the response to treatment in bacterial meningitis.
Keywords: Procalcitonin, Meningitis, Pediatrics
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Lecturer, Department of Community Medicine, Govt. Medical College, Srinagar, Jammu and Kashmir, India. Email: |
Ahmad M, Iqbal J, Wani FA, Ali SW. Role of serum procalcitonin in monitoring the response to treatment of pediatric meningitis. Pediatric Rev Int J Pediatr Res. 2020;7(7):351-355. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/626 |


 ©
©