Comparison of intravenous Paracetamol with oral Ibuprofen for medical closure of PDA (patent ductus arteriosus) in preterm newborns – can it be an effective, safe and preferable choice?
Kumar Rathia S.1, Kumar Kurrey V.2*, Shrivastava S.3, Kumar Gupta A.4, Phuljhele S.5
DOI: https://doi.org/10.17511/ijpr.2021.i01.05
1 Santosh Kumar Rathia, Assistant Professor, Department of Trauma & Emergency – Pediatrics, All India Institute of Medical Sciences, Raipur, Chhattisgarh, India. 2* Virendra Kumar Kurrey, Associate Professor, 3 Smit Shrivastava, Associate Professor, department of Cardiology, 4 Ankit Kumar Gupta, Senior Resident, 5 Sharja Phuljhele, Professor; all authors are affiliated with the Department of Pediatrics, Pt. J. N. M. Medical College, Raipur, Chhattisgarh, India.
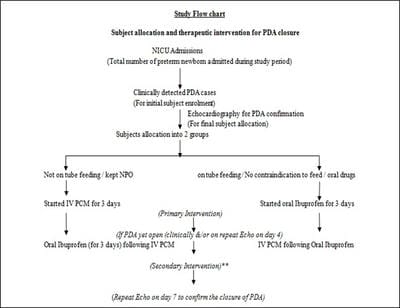
Introduction: In prematurely born newborns, the ductus arteriosus frequently fails to close and more than half of preterms might have Patent Ductus Arteriosus (PDA) after birth during the first 24hours. About 70% of infants born before 28 weeks may require medical or surgical closure of PDA. Objectives: To compare the effect of i/v PCM and oral Ibuprofen on PDA closure rate in preterm neonates with a gestational age of < 37 weeks and evaluate the side effect profile of both drugs. Material and Methods: This time-bound prospective observational study for comparison of the efficacy of the two drugs on the closure of PDA in preterms admitted during 1.5 years of the study period in a neonatal ICU caring for inborn as well as outborn neonates in central India. After approval of the institutional ethics committee, initial clinical screening included all preterms but only those with PDA (open ductus arteriosus) confirmed by 2D-echocardiography were allocated to receive either of two treatment regimens (3-day courses of each oral Ibuprofen and i/v PCM) which already exist as evidence-based therapeutic practice options. Result: Out of a total of 43 cases clinically suspected on initial screening, 30 preterm babies confirmed by 2D-Echo to have PDA were finally enrolled and analysed. Twenty-one patients received i/v PCM with a resultant PDA closure rate of 85.7 % (n = 18/21), while nine cases could be allocated oral Ibuprofen arm and ductus closure was obtained in seven patients (77.78%). Conclusion: Paracetamol, preferably by the intravenous route, seems to be an effective, cheap, easily available and safe first-line alternative to oral Ibuprofen for the closure of PDA, especially in newborns with extreme prematurity, who are initially intolerant to oral feeds or drugs.
Keywords: Preterm, PDA, Medical closure, Ibuprofen, Paracetamol, Intravenous
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pediatrics, Pt. J. N. M. Medical College, Raipur, Chhattisgarh, India. Email: |
Rathia SK, Kurrey VK, Shrivastava S, Gupta AK, Phuljhele S. Comparison of intravenous Paracetamol with oral Ibuprofen for medical closure of PDA (patent ductus arteriosus) in preterm newborns – can it be an effective, safe and preferable choice?. Pediatric Rev Int J Pediatr Res. 2021;8(1):29-38. Available From https://pediatrics.medresearch.in/ index.php/ijpr/article/view/639 |


 ©
©