“Study of Estimation of Cerebrospinal Fluid C-Reactive Protein in Diagnosis of Acute Meningitis.”
Bansal K.1, Bhatt D.2*, Kumar Dadhich S.3, Kariya D.4
DOI: https://doi.org/10.17511/ijpr.2021.i04.02
1 Keshav Bansal, Senior Resident, Department of Pediatrics, Pacific Institute of Medical Science, Udaipur, Rajasthan, India.
2* Dhaval Bhatt, Assistant Professor, Department of Pediatrics, Government Medical College, Bhavnagar, Gujarat, India.
3 Sunil Kumar Dadhich, Assistant Professor, Department of Pediatrics, Government Medical College, Kota, Rajasthan, India.
4 Deep Kariya, PG Resident, Department of Pediatrics, Government Medical College, Bhavnagar, Gujarat, India.
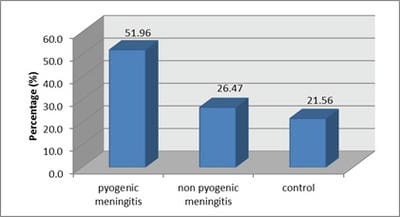
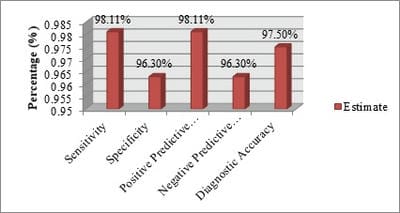
Objectives:To assess the diagnostic role of CSF C-reactive protein quantitatively in acute meningitis and to evaluate the efficacy of CSF C-reactive protein in differentiating pyogenic meningitis from non-pyogenic meningitis.Material and Methods: It is a prospective observational study of total 102 children with suspected meningitis allocated into three groups based on initial investigations; group-I Pyogenic meningitis, group-II Non-Pyogenic meningitis and group-III No meningitis (Control group). Quantitative CSF C-reactive protein was detected by the latex agglutination method. Data were analyzed to establish the diagnostic role of CSF-CRP and to evaluate the efficacy of CSF-CRP in differentiating pyogenic meningitis from non-pyogenic meningitis.Results: A total of 102 clinically suspected meningitis patients were studied. Based on CSF findings, the study population (102 cases) was categorized into 3 groups. Group I was pyogenic meningitis consist 53 cases (51.96%). Group II was Non-Pyogenic meningitis consists 27 cases (26.47%). Group III was normal CSF findings consist 22 (21.56%). 98.1% cases of pyogenic meningitis had elevated CSF-CRP level >1.1 µg/ml of CSF. In the case of Non-Pyogenic meningitis, 96.2% were found to have CSF- CRP in the range of 0.05-0.10 µg/ml. The mean value of CSF-CRP in groups I,II and III were 5.57±1.48, 0.09±0.042 and 0.01±0.010 respectively. Conclusion: Detection of CSF-CRP provides a new dimension to establish the diagnosis of pyogenic meningitis. It is a rapid, reliable and sensitive diagnostic test. From this study it is concluded that CSF-CRP can be used to differentiate pyogenic from non-pyogenic meningitis. Early, accurate and appropriate therapy can ameliorate the morbidity and mortality rates in such cases.
Keywords: CSF-CRP, Meningitis, Brain Infections
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Pediatrics, Government Medical College, Bhavnagar, Gujarat, India. Email: |
Bansal K, Bhatt D, Dadhich SK, Kariya D. “Study of Estimation of Cerebrospinal Fluid C-Reactive Protein in Diagnosis of Acute Meningitis.”. Pediatric Rev Int J Pediatr Res. 2021;8(4):175-181. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/660 |


 ©
©