Hemophilia in children: a clinico-epidemiological profile and review
Singh M.1, Gupta L.2*, Aneja G.3, Dayal R.4, Kumar N.5, Singh S.6, Nayak M.7, Kshitiz Sharma R.8
DOI: https://doi.org/10.17511/ijpr.2020.i02.04
1Madhu Singh, Assistant Professor, 2*L.K. Gupta, Associate Professor, Department of Pediatrics, Government Medical College, Firozabad, Uttar Pradesh, India. 3G.K. Aneja, D.M. Cardiology, Professor and Dean, 4R. Dayal, Professor and Head, 5 Neeraj Kumar, Professor, 6Sheo Pratap Singh, Assistant Professor, 7Madhu Nayak, Assistant Professor, 8Ram Kshitiz Sharma, Assistant Professor; 1,3,4,5,6,7,8authors are affiliated with the Department of Paediatrics, S.N. Medical College, Agra, Uttar Pradesh, India.
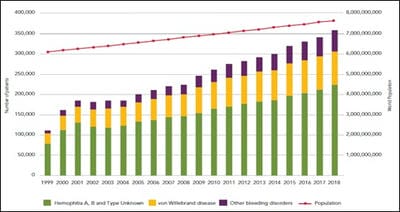
Introduction: Objective: To describe the clinical and epidemiological profile of hemophilia patients in the pediatric age group at a tertiary care center in Western Uttar Pradesh. Materials and Methods: This was an observational cross-sectional study that was conducted at the Department of Pediatrics, S.N. Medical College, Agra. Pediatric patients (0-18 years) with hemophilia admitted in the Pediatrics emergency ward for Anti- Hemophilic factor transfusion or having a history of such type of transfusion during the year 2018-19 were consecutively enrolled in the present study. A detailed clinical history was taken from the parent or guardian accompanying the case. Results: Out of the total 93 pediatric hemophilia patients, the majority 61% (57/93) belonged to 11 to 18 years of age group. 2.1% (2/93) were< 1 year of age. 17.2 % (16/93) were in 1-5 years of age group. 19.35% were in 6-10 years of age group. Out of the total, 98.9% (92/93) were males while only 1.07% (1/93) were female. Type A hemophilia was observed in 87.09% (81/93) patients while Type B hemophilia was present in only 12.9% (12/93) patients. Out of 81 Type-A hemophilia patients, only one had developed Inhibitor (1.07%). None among Type B hemophilia patients had Inhibitors. Out of total cases, only one patient (1.07%) had von Willebrand Deficiency.91%(85/93) patients presented with joint bleed. The knee joint was the most commonly affected joint, as observed in 64.7% (55/85) patients. Conclusion: A better insight into the disease prevalence is pivotal for early diagnosis and to provide quality care to all hemophilia patients. The development of an inhibitor is a major concern. Physiotherapy and RICE(Rest, Ice, Compression and elevation) should be emphasized for the better quality of life and preventing joint hemarthrosis.
Keywords: Clinico- epidemiological profile, Hemophilia, Inhibitors, Rest, Ice, Compression and Elevation (RICE)
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department of Pediatrics, Government Medical College, Firozabad, Uttar Pradesh, India. Email: |
Singh M, Gupta LK, Aneja GK, Dayal R, Kumar N, Singh SP, Nayak M, Sharma RK. Hemophilia in children: a clinico-epidemiological profile and review. Pediatric Rev Int J Pediatr Res. 2020;7(2):66-72. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/575 |


 ©
©