Organisms causing urinary tract infections in children and their sensitivity pattern in a level 2 pediatric hospital at a district place in South India
Marol R.1*, kumar Marol R.2, Marol R.3
DOI: https://doi.org/10.17511/ijpr.2020.i03.03
1* Rajakumar Marol, Senior Consultant, Department of Pediatrics, Shivajyoti Institute of Child Health, Haveri, Karnataka, India.
2 Rohit kumar Marol, Research Assistant, Department of Pediatrics, Shivajyoti Institute of Child Health, Haveri, Karnataka, India.
3 Renuka Marol, Pathologist, Department of Pediatrics, Shivajyoti Institute of Child Health, Haveri, Karnataka, India.
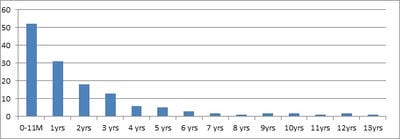
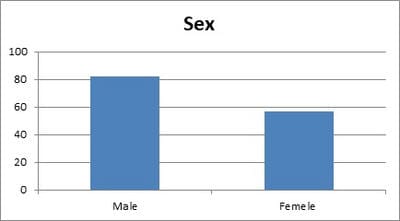
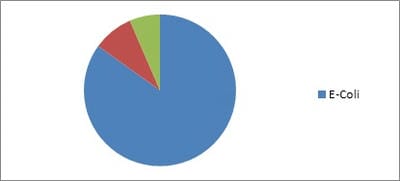
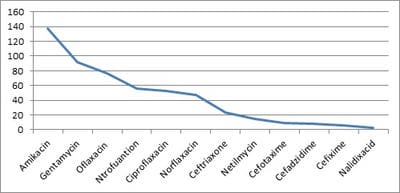
Background: Urinary Tract Infection is second the common bacterial infection affecting children. Resistance to routine antibiotics for treating CA-UTI is on the rise. It is necessary to determine the antibiotic sensitivity patterns of uropathogensin a particular region to prevent complications of UTI and to determine empirical antibiotic. Aims: To evaluate the bacteriological profile and antibiotic sensitivity patterns in children with urinary tract infections Setting and Design: Retrospective observational study conducted over a period of 2 years in a level 2 pediatric hospital at a district place in South India Materials and methods: Children between the ages of one day and 13 years who had UTI over a period of 2 years from 01 Jan 2018 to 31 Dec 2019 were included in the study. Urine specimens with the growth of single organism of >105/ml were studied forvariousuropathogens and their sensitivity patterns. Statistical analysis: All the data obtained were presented in percentages and frequencies using Microsoft excel. Results: Out of 446 urine samples, 139 children were positive for pathogenic organisms. Escherichia coli constituted for 118 (84%) organisms followed by Proteus Mb 12 (8.6%) and Klebsiella sp 9 (6.4%).The organisms were found to be most sensitive to Amikacin (98%), Gentamicin (66%), and Ofloxacin (55%.). Conclusion: E.coli is the leading pathogen causing UTI in children. It is highly sensitive to amikacin and is resistant to most of the commonly used antibiotics. This knowledge is necessary to treat UTI empirically in the local population.
Keywords: UTI, Children, Uropathogens, Antibiotics, Sensitivity
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Consultant, Department of Pediatrics, Shivajyoti Institute of Child Health, Haveri, Karnataka, India. Email: |
Marol R, Marol RK, Marol R, Organisms causing urinary tract infections in children and their sensitivity pattern in a level 2 pediatric hospital at a district place in South India. Pediatric Rev Int J Pediatr Res. 2020;7(3):129-135. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/587 |


 ©
©