Thyroid profile in patients of thalassemia with multiple blood transfusions and high serum ferritin: a cross-sectional study
Khandelwal R.1, Gundluru M.2*, Mehta K. L.3
DOI: https://doi.org/10.17511/ijpr.2020.i08.02
1 Rohit Khandelwal, Associate Professor, Department of Paediatrics, Vydehi Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India.
2* Muralidhar Gundluru, Assistant Professor, Department of Paediatrics, Vydehi Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India.
3 Leeni Mehta K., Consultant Physician, Apollo Group of Hospitals, Bangalore, Karnataka, India.
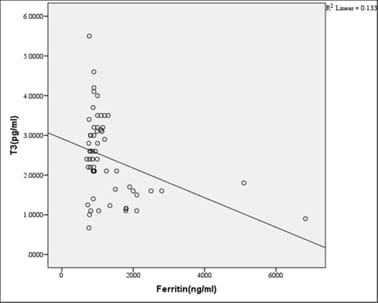
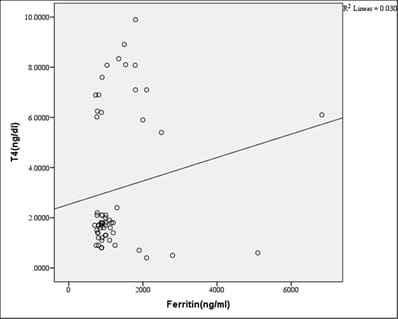
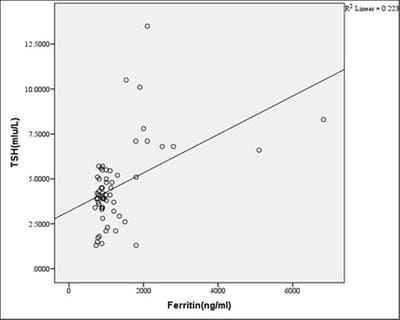
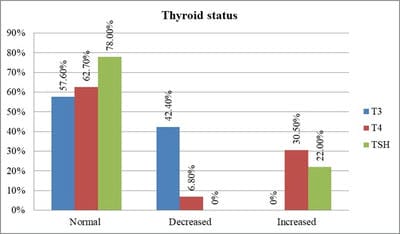
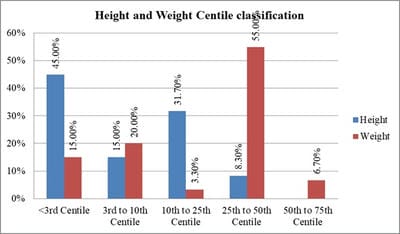
Introduction: Beta-thalassemia major patients undergo regular blood transfusion resulting in growth faltering and various endocrine problems including thyroid dysfunction due to iron overload in the body. This study was conducted to determine the frequency of thyroid dysfunction in children presenting with Beta-thalassemia major on regular blood transfusions. Materials and methods: Sixty children were included with proven beta-thalassemia major who reported to the Department of Pediatrics, VIMS, and RC, Bangalore. Inclusion criteria: 1.Children 4 to 18 years age group .2.The child received transfusions for more than 2 years. 3.Children with serum ferritin level >700. Results: In this study, four patients(6.8%) had overt hypothyroidism, eight patients(13.6%) had subclinical hypothyroidism and 47 patients(79.7%) had euthyroid status. There was a positive correlation between Ferritin and T4, TSH levels. i.e., with an increase in Ferritin level, there was an increase in T4, TSH levels, and vice versa. However, the correlation was significant with TSH. There was a significant negative correlation between Ferritin and T3 levels. i.e with an increase in Ferritin level, there was a decrease in T3 levels and vice versa. Conclusion: Thyroid dysfunction can exist in thalassemia patients on multiple transfusions and chelation therapy with high serum ferritin levels. Detection of hypothyroidism is important as inexpensive oral replacement therapy is readily available. Hence regular screening of beta-thalassemia major patients for Serum T3, Serum T4, Serum TSH for early detection and timely treatment could improve the life expectancy and quality of life of these patients.
Keywords: Blood transfusion, Hypothyroidism, Serum Ferritin, Serum T3, Serum T4, Serum TSH, Thalassemia
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Paediatrics, Vydehi Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India. Email: |
Khandelwal R, Gundluru M, Mehta KL. Thyroid profile in patients of thalassemia with multiple blood transfusions and high serum ferritin: a cross-sectional study. Pediatric Rev Int J Pediatr Res. 2020;7(8):401-408. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/646 |


 ©
©