Clinical biochemical and serological profile in Children with Celiac disease
Shrivastava V.1, Lohia P.2*
DOI: https://doi.org/10.17511/ijpr.2021.i03.01
1 Vishal Shrivastava, Department of Pediatrics, People’s College of Medical Sciences & Research Centre, Bhopal, Madhya Pradesh, India.
2* Purnendu Shekhar Lohia, Assistant Professor, Department of Paediatrics, People’s College of Medical Sciences and Research Centre, Bhopal, Madhya Pradesh, India.
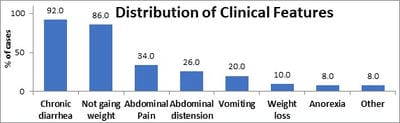
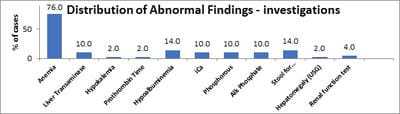
Background: Celiac disease (CD) is a chronic, immunologically determined form of enteropathy affecting the small intestine, precipitated by the ingestion of gluten-containing foods such as wheat rye, barley etc. This study was taken up to analyze clinical manifestations and biochemical profile of children with celiac disease presenting at KEM Hospital Pune. Methods: All children diagnosed as CD in last 5 years and newly diagnosed patients of celiac satisfying inclusion criteria for next 1 year. This is an observational descriptive prospective and retrospective study. CD was diagnosed based on positive tTGA & duodenal biopsy in children with chronic diarrhea & other suggestive features. Hospital records were reviewed for complete follow up data. Results: In a study period of 12 months we diagnosed 19 children with Celiac Disease, who were studied prospectively, whereas 31 patients who were diagnosed within the last 6 years & were on regular follow up in OPD were studied retrospectively. In the total group of 50 patients in the age range of 1year to 15 years. The presenting clinical features of our group of patients were: chronic diarrhea (92 %), failure to thrive (86%), abdominal pain (34%), abdominal distention (26%), anorexia/vomiting (8%/ 20%), & weight loss (8%). Rare features were fever, fatigue, blood in stools & constipation. In our study anemia was seen in 76% of patients, 58% of prospective patients had low ferritin levels. Conclusions: In a study period of 12 months we diagnosed 19 children with Celiac disease which goes to go prove that Celiac Disease, is not rare in western Maharashtra. Most patients belonged to the age group 1-5 years (50%) and the majority of patients were females (56%). Chronic diarrhea was the most common presenting complaint in all age groups ( 92%) followed by failure to thrive, not gaining weight and abdominal pain. Constipation was least common. Anemia was the most common laboratory-confirmed finding and the most common type of anemia was iron deficiency anemia. Prevalence Anemia was most common in below 5 yrs.
Keywords: Celiac, Chronic diarrhea, Anemia
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Paediatrics, People’s College of Medical Sciences and Research Centre, Bhopal, Madhya Pradesh, India. Email: |
Shrivastava V, Lohia PS. Clinical biochemical and serological profile in Children with Celiac disease. Pediatric Rev Int J Pediatr Res. 2021;8(3):121-129. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/676 |


 ©
©