Evaluation of Thyroid Hormone Levels in Full-Term Neonates Presented with Septic Shock
Singh D.1*, Shrivastava J.2, Agrawal A.3
DOI: https://doi.org/10.17511/ijpr.2021.i04.01
1* Dhananjay Singh, Senior Resident, Department of Pediatrics, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
2 Jyotsana Shrivastava, Professor and HOD, Department of Pediatrics, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
3 Amit Agrawal, Associate Professor, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
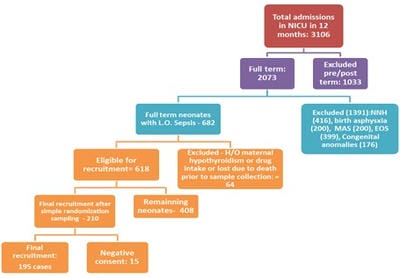
Introduction: The incidence of neonatal sepsis in India is 38 per 1000 live births. Many authors found an association between altered thyroid hormone levels and septic shock in neonates and it may be of prognostic importance in septic shock treatment. This study has been conducted to find the relationship between thyroid profile and septic shock in neonates and also to compare the thyroid profile in survivor and non-survivor groups of septic shock patients. Methods: This analytical prospective cohort study was conducted in the NICU of a tertiary care teaching institution in central India. Full-term neonates with late-onset sepsis were included in this study and estimation of thyroid hormones (TSH, T3, T4, fT3, and fT4) was performed. These neonates were divided into those with and without septic shock patients and levels of thyroid hormones were correlated between these patients to find significant relations. The Vasoactive-Inotropic Score (VIS) score was calculated. Results: A total of 195 full-term neonates were included in the study. The mean value of TSH, T3, T4, fT3, and fT4 among neonates with septic shock were 5.27 µg/ml, 80.01 ng/dl, 6.36 µg/dl, 1.40 pg/ml, and 1.40 µg/dl, respectively while the values were 5.29 µg/ml, 94.4 ng/dl, 7.25 µg/dl, 1.84 pg/ml, and 1.43 µg/dl, respectively in septic neonates without shock. This difference was statistically significant except for TSH (p>0.05). The mean value of TSH, T3, T4, fT3, and fT4 among septic shock survivors were 5.27 µg/ml, 80.01 ng/dl, 6.36 µg/dl, 1.40 pg/ml, and 1.40 µg/dl and in septic shock non-survivors were 2.40 µg/ml, 37.33 ng/dl, 3.86 µg/dl, 0.99 pg/ml, and 0.84 µg/dl, respectively (p<0.0001). Only T3 was found to be significantly co-related with VIS in septic shock in all the groups (<0.001). Conclusion: Our study suggests that TSH, T3, T4, fT3, and fT4 levels are significantly low in patients suffering from the septic shock which may vary in the case of TSH. Also, there is a significant decrease in thyroid profile among septic shock non-survivors as compared to survivors.
Keywords: Neonate, Thyroid profile, Septic shock, Survivors
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Senior Resident, Department of Pediatrics, Gandhi Medical College, Bhopal, Madhya Pradesh, India. Email: |
Singh D, Shrivastava J, Agrawal A. Evaluation of Thyroid Hormone Levels in Full-Term Neonates Presented with Septic Shock. Pediatric Rev Int J Pediatr Res. 2021;8(4):168-174. Available From https://pediatrics.medresearch.in/index.php/ijpr/article/view/683 |


 ©
©