A study to compare the pulmonary function test in non-asthmatic allergic and non-asthmatic non-allergic (Apparently Healthy) children-in age group 6-12 years
Abstract
Aims & Objective: To compare Pulmonary Function Test in Non-Asthmatic Allergic and Non-Asthmatic Non-Allergic children.
Background: Many studies have shown that Allergic children are prone to develop Asthma or may have subclinical symptoms of asthma. By comparing pulmonary function test in Allergic children and Non-Allergic children we tried to find out impact of allergy on pulmonary function. We compared the pulmonary functions in the healthy Allergicand Non-Asthmatic children.
Method: This is cross sectional observational case-control study. All 75 children who were selected randomly, in pediatric Out Patient Department (OPD) undergoneBurlingtonent Clinical Questionnaire scoring system, for the clinical assessment of possibility/probability of Allergy (score more than 8). Then, all were evaluated through pulmonary function test by Spirometry. Data of Premedication and Post medication Pulmonary Function parameters were collected and have been compared in relation to Allergic and Non-Allergic possibility. Result were expressed as Mean± SD (standard deviation) and p value was calculated with student paired ‘t’ test and unpaired ‘t’ test.
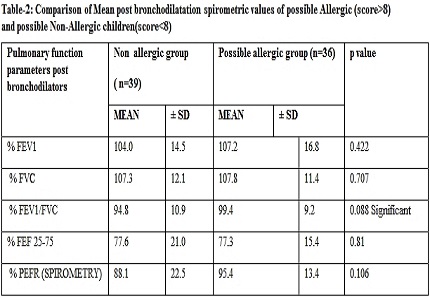
Results: In our study, statistically significant difference (p<0.01) was found in Pre-bronchodilator Pulmonary Function parameters (%FVC & %FEF25-75) among possible Allergic & possible Non-Allergic group of children, which, however, was non-significant in post bronchodilator Pulmonary function test. On comparing the pre and post medication pulmonary function test parameters (% FEV1, % FVC, % FEV1/FVC, % FEF 25-75, % PEFR(SPIROMETRY), there was statistically significant (p<0.01) positive increment in all these individual lung spirometry parameters in possible Allergic group and not in healthy control group. Although the spirometric parameters did not fulfill the criteria of Asthma, but small airway function parameters were abnormal among possible allergic children.
Conclusion: There is some correlation between clinical suspicion of Allergy and changes in lung parameters in Pulmonary Function Test, which may favoroccurrence of future lung disease and the potential of reversibility in allergic groups by appropriate therapeutic guidance.
Downloads
References
2. Wallace DV, Dykewicz MS, Bernstein DI, et al.Joint Task Force on Practice; American Academy of Allergy; Asthma & Immunology; American College of Allergy; Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008 Aug;122(2 Suppl):S1-84. doi: 10.1016/j.jaci.2008.06.003. [PubMed]
3. Pawankar R, CanonicaGw, Holgate St Et Al. World Allergy Organization White Book On Allergy 2011-2012 Executive Summary. World Allergy Organization.
4. Douglas E. Henrich, M.D. Jennifer K. Berge, M.D. Burlington Ear, Nose &Throat, P.C. West Burlington Iowa Http://Www.Burlingtonent.Com/Files/Allergy-Questionnaire.Pdf West Burlington Lowa1225 South Gear,Avenuesuite 255, West Burlington, Iowa, 52655
5. Lombardi C1, Gani F, Landi M, Boner A, CanonicaGw, Passalacqua G,Clinical and Therapeutic Aspects Of possible Allergic Asthma In Adolescents. Pediatr Allergy Immunol. 2003 Dec; 14(6):453-7.
6. Bousquet J, Van Cauwenberge P, Khaltaev N; Aria Workshop Group; World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001 Nov;108(5 Suppl):S147-334.
7. Grossman J. One airway, one disease. Chest. 1997 Feb;111(2 Suppl):11S-16S. [PubMed]
8. Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001 Feb;1(1):7-13. [PubMed]
9. Rajesh tikkas,Lokendra Dave, Priyanka Choudhary et al. A study of allergic sensitization to eight common allergens in pediatric patients with nasobronchial allergy. Journal of evolution of medical and dental sciences 2015; 4(63):11001-11007
10. Gildea TR, McCarthy K. Pulmonary Function Testing Clevland Clinic Disease management project, January ,2009, on line
11.Cochrane GM, Prieto F, Clark TJ. Intrasubject variability of maximal expiratory flow volume curve. Thorax. 1977 Apr;32(2):171-6. [PubMed]
12. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983 Jun;127(6):725-34. [PubMed]
13. Eggleston PA. Upperairwayinflammatorydiseases and bronchial hyperresponsiveness. J Allergy Clin Immunol.1988 May;81(5Pt2):1036-41. [PubMed]
14. Samoliński B, Szczesnowicz-Dabrowska P. Relationship between Inflammation of Upper and Lower Respiratory Airways. Otolaryngol Pol. 2002; 56(1):49-55.
15. Chhabra SK, Gupta CK, Chhabra P, Rajpal S. Prevalence of bronchial asthma in schoolchildren in Delhi. J Asthma. 1998;35(3):291-6. [PubMed]
16. Chhabra SK, Anonymous, All India Coordinated Project on Aeroallergens and Human Health Report. Ministry of Environment and Forests, New Delhi; 2000.
17. Bavbek S, Saryal S, Karabiyikoglu G, Misirligil Z. Pulmonary function parameters in patients with allergic rhinitis. J Investig Allergol Clin Immunol. 2003;13(4):252-8. [PubMed]
18. Ciprandi G1, Ricciardolo FL, Schiavetti I, Cirillo I. possible Allergic Rhinitis Phenotypes Based on bronchial hyperreactivity to Methacholine. Am J Rhinol Allergy. 2014 Nov; 28(6):214-8. Doi: 10.2500/Ajra.2014.28.4124.
Copyright (c) 2018 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


