To compare the efficacy of fortification of expressed breast milk with medium chain triglycerides and coconut oil on the physical growth of very low birth weight babies
Abstract
Background: The most important factor leading to growth failure in VLBW babies is inadequate nutrition. Though, higher amounts of protein, sodium, chloride, and magnesium are available in preterm human milk than those found in term milk, these levels still remain below recommendations. To compensate these requirements, human milk for preterm infants is routinely fortified to increase energy, protein and mineral intake. In the past, very few studies have been done to find out the efficacy of medium chain triglycerides and coconut oil for fortification of human milk. Since, a major chunk of LBW babies exists in our country, this study is undertaken.
Objectives: To study and compare the physical growth (weight, length and head circumference) over a period of two months in each study group.
Design: Prospective cohort study.
Setting: The study was conducted from April, 2015 to August, 2016 at Department of Pediatrics, MY hospital and CNBC, Indore.
Participants: 225 VLBW babies (<1.5 kg) admitted in SCNU and postnatal wards.
Intervention: The babies were assigned to group 1, 2 and 3 in serial order. Sample size in each group was 75.
Group1- Given expressed breast milk with lactodex-HMF.
Group 2- Given expressed breast milk with lactodex- HMF and MCT (Simyl MCT oil).
Group 3- Given expressed breast milk with lactodex- HMF and coconut oil (Parachute-100% edible oil) Physical growth was monitored every week for 2 months.
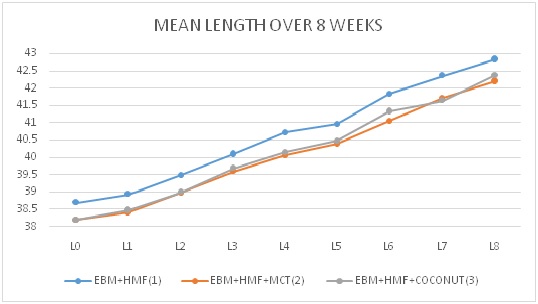
Results: Significant physical growth (weight, length and head circumference) was noticed in each of the study groups over 2 months (p value<0.05). There was no statistically significant difference in the physical growth between the groups (p>0.05).
Conclusion: There is no advantage of MCT or coconut oil in addition to Lactodex- HMF on the physical growth of VLBW babies.
Downloads
References
2. Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001 Feb;107(2):270-3.[pubmed]
3.McGuire W, Henderson G, Fowlie PW. Feeding the preterm infant. BMJ. 2004 Nov 20;329(7476):1227-30. [pubmed]
4. Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. J Nutr. 1995 Aug;125(8 Suppl):2233S-2238S. doi: 10.1093/jn/125.suppl_8.2233S.doi:10.1093/jn/125.suppl_8.2233S. [pubmed]
5. Cooper PA, Rothberg AD, Pettifor JM, et al. Growth and biochemical response of premature infants fed pooled preterm milk or special formula. J Pediatr Gastroenterol Nutr. 1984 Nov;3(5):749-54.[pubmed]
6. Davies DP. Adequacy of expressed breast milk for early growth of preterm infants. Arch Dis Child. 1977 Apr;52(4):296-301..[pubmed]
7. Kashyap S, Schulze KF, Forsyth M, et al. Growth, nutrient retention, and metabolic response of low-birth-weight infants fed supplemented and un supplemented preterm human milk. Am J Clin Nutr. 1990 Aug;52(2):254-62.doi:10.1093/ajcn/52.2.254.[pubmed]
8. Andrews BF, Lorch V: Improved fat and Ca absorption in L.B.W. infants fed a medium chain triglyceride containing formula. Pediatric Research.1974; 8(4):378
9. Tantibhedhyangkul P, Hashim SA: Clinical and physiologic aspects of medium-chain triglycerides: Alleviation of steatorrhea in premature infants. Bull NY Acad. Med.1971 Jan;47(1):17-33
10. Tantibhedhyangkul P, Hashim SA: Medium-chain triglyceride feeding in Premature infants: Effects on fat and nitrogen absorption. Pediatrics.1975 Mar; 55(3):35
11. Andrews BF, Lorch V: Improved fat and Ca absorption in L.B.W infants fed a medium chain triglyceride containing formula. Pediatric Research.1974; 8(4):378
12. Roy CC, Ste-Marie M, Chartrand L, et al. Correction of the malabsorption of the preterm infant with a medium-chain triglyceride formula. J Pediatr. 1975 Mar;86(3):446-50.[pubmed]
13.O’ Connor DL, Hall R, Adamkin D, et al. Growth and development in preterm infants fed long chain polyunsaturated fatty acids: a prospective randomized control trial. Pediatrics.2001 Aug;108(2):359-71.[pubmed]
14. Fewtrell MS, Abbott RA, Kennedy K, et al. Randomized double blind trial of long chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. J Pediatr. 2004 Apr; 144(4):471-9.[pubmed]
15.Collins CT, Makrides M, Gibson RA et al. Pre and post term growth in preterm infants supplemented with higher dose DHA: a randomised control trial. Br J Nutr. 2011 Jun;105(11):1635-43.doi:10.1017/S000711451000509X.[pubmed]
16.O’Connor DL,Khan S et al.Growth and nutrient intakes of human milk fed preterm infants provided with extra energy and nutrients after hospital discharge.Pediatrics.2008 Apr;121(4):766-76.doi:10.1542/peds.2007-0054.[pubmed]
17. Deborah L. O’ Connor, Sobia Khan, Karen Weishuhn, Jennifer Vaughan et.al. Growth and Nutrient Intakes of Human Milk- Fed Preterm Infants provided with extra energy and nutrients after discharge. Pediatrics.2008 Apr;121(4);766-76.doi:10.1542/peds.2007-0054
18. Vaidya UV, Hegde VM, Bhave SA, Pandit AN. Vegetable oil fortified feeds in the nutrition of very low birthweight babies. Indian Pediatr. 1992 Dec;29(12):1519-27.[pubmed]
19.Klenoff Brumberg HL, Genen LH et al. High versus low medium chain triglyceride content of formula for promoting short term growth of preterm infants. Cochrane Database Syst Rev.2003;(1):CD002777
20. Amissah EA, Brown J et al. Fat supplementation of human milk for promoting growth in preterm infants. Cochrane Database Syst Rev.2018 Jun 19;6:CD000341.doi:10.1002/14651858.CD000341.pub2.
Copyright (c) 2018 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


