Transcatheter closure of patent ductus arteriosus in children weighing 5 kg or less: Initial experienc
Abstract
Introduction: Trans-catheter closure (TCC) of patent ductus arteriosus (PDAs) is a well-establishednon-surgical method of closure.
Objectives: To evaluate safety, feasibility & complicationsof device closure of patent ductus arteriosus (PDA) in small children weighing ≤ 5 kg with different types of devices for closure.
Methods: patients with PDA underwent transcatheter closure, among whom 16 (33%) weighed 5 kg or less. All of these patients underwent transcatheter closure of PDA using an Amplatzer duct occluder I, Amplatzer duct occluder II or Amplatzer duct occluder 2 IIAS (Additional size) devices.
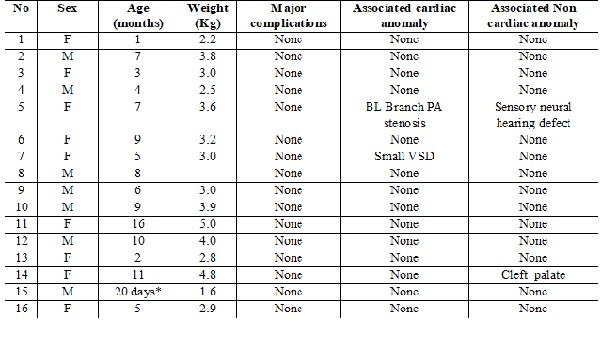
Results: Sixteen patients (9 females & 7 males) between theages 20 days to 16 months had undergone device closures. Amplatzer Duct Occluder (ADOI) was used in 12 cases, 3 were closed with Amplatzer Duct Occluder (ADO2) and one the smallest of all (1.6 kg) patientwere selected for closure with Amplatzer Duct Occluder (ADO2 AS).At 1 month after the closure, the signs and symptoms improved markedly in all patients, and PDAs were completely closed and devices remained in situ on follow-up. Mild obstruction of left pulmonary artery (n=2) and aortic isthmus flow (n=1) was noted at the time of discharge which gradually improved on follow up.
Conclusions: TCC of PDAin small babies 5 kg or less is feasible & safe alternative to surgical PDA closure in carefully selected patients. The immediate & short-term outcomes have proven this method to be safe & valid.
Downloads
References
2. Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. 1971 Mar;43(3):323-32.[pubmed]
3. Krichenko A, Benson LN, Burrows P, et al. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989 Apr 1;63(12):877-80.[pubmed]
4. Masura J, Walsh KP, Thanopoulous B, et al. Catheter closure of moderate- to large-sized patent ductus arteriosus using the new Amplatzer duct occluder: immediate and short-term results. J Am Coll Cardiol. 1998 Mar 15;31(4):878-82.[pubmed]
5. Balzer DT, Kort HW, Day RW, et al. Inhaled Nitric Oxide as a Preoperative Test (INOP Test I): the INOP Test Study Group. Circulation. 2002 Sep 24;106(12 Suppl 1):I76-81.[pubmed]
6. McLaughlin P, Benson L, Horlick E. The role of cardiac catheterization in adult congenital heart disease. Cardiol Clin. 2006 Nov;24(4):531-56, v. DOI:10.1016/j.ccl.2006.08.008
7. Viswanathan S, Kumar RK. Assessment of operability of congenital cardiac shunts with increased pulmonary vascular resistance. Catheter Cardiovasc Interv. 2008 Apr 1;71(5):665-70. doi: 10.1002/ccd.21446.[pubmed]
8. Thanopoulos BD, Tsaousis GS, Djukic M, et al. Transcatheter closure of high pulmonary artery pressure persistent ductus arteriosus with the Amplatzer muscular ventricular septal defect occluder. DOI:10.1136/ heart.87.3.260
9. Roy A, Juneja R, Saxena A. Use of Amplatzer duct occluder to close severely hypertensive ducts: utility of transient balloon occlusion. Indian Heart J. 2005 Jul-Aug;57(4):332-6.[pubmed]
10. Anil SR, Sivakumar K, Philip AK, Francis E, Kumar RK. Clinical course and management strategies for hemolysis after trans catheter closure of patent ductus arteriosus. Catheter Cardiovasc Interve2003;59(4):538-43
11. Celiker A, Aypar E, Karagöz T, et al. Transcatheter closure of patent ductus arteriosus with Nit-Occlud coils. Catheter Cardiovasc Interv. 2005 Aug;65(4):569-76.[pubmed]
12. Fortescue EB, Lock JE, Galvin T, et al. To close or not to close: the very small patent ductus arteriosus. Congenit Heart Dis. 2010 Jul-Aug;5(4):354-65. doi: 10.1111/j.1747-0803.2010.00435.x.[pubmed]
13. Jan SL, Hwang B, Fu YC, et al. Transcatheter closure of a large patent ductus arteriosus in a young child using the Amplatzer duct occluder. PediatrCardiol. 2005 Sep-Oct;26(5):703-6. DOI:10.1007/s00246-004-0894-z.
14. Choi DY, Kim NY, Jung MJ, et al. The results of transcatheter occlusion of patent ductus arteriosus: success rate and complications over 12 years in a single center. Korean Circ J. 2010 May;40(5):230-4. doi: 10.4070/kcj.2010.40.5.230. Epub 2010 May 27.
15. Al-Ata J, Arfi AM, Hussain A, et al. The efficacy and safety of the Amplatzer ductal occluder in young children and infants. Cardiol Young. 2005 Jun;15(3):279-85. DOI:10.1017/S1047951105000570
16. Butera G, De Rosa G, Chessa M, et al. Transcatheter closure of persistent ductus arteriosus with the Amplatzer duct occluder in very young symptomatic children. Heart. 2004 Dec;90(12):1467-70. DOI:10.1136/hrt.2003.025122
17. Park YA, Kim NK, Park SJ, et al. Clinical outcome of transcatheter closure of patent ductus arteriosus in small children weighing 10 kg or less. Korean J Pediatr. 2010 Dec;53(12):1012-7. doi: 10.3345/kjp.2010.53. 12.1012. Epub 2010 Dec 31.
18. El-Said HG, Bratincsak A, Foerster SR, Murphy JJ, Vincent J, Holzer R, et al. Safety of percutaneous patent ductus arteriosus closure: an unselected multicenter population experience. J Am Heart Assoc 2013;2(6):e000424.[pubmed]
19. Dimas VV, Takao C, Ing FF, Mattamal R, Nugent AW, Grifka RG, et al. Outcomes of transcatheter occlusion of patent ductus arteriosus in infants weighing ≤6kg. JACC Cardiovasc Interv. 2010;3(12):1295–9.
20. Wang JK, Wu MH, Hwang JJ, et al. Transcatheter closure of moderate to large patent ductus arteriosus with the Amplatzer duct occluder. Catheter Cardiovasc Interv. 2007 Mar 1;69(4):572-8.[pubmed]
21. Tometzki AJ, Arnold R, Peart I, Sreeram N, Abdulhamed JM, odman MJ, et al. Transcatheter occlusion of the patent ductus arteriosus with Cook detachable coils. Heart 1996;76:531–535.
22. Behjati-Ardakani M, Behjati-Ardakani MA, Hosseini SH, et al. Long-term results of transcatheter closure of patent ductus arteriosus in infants using amplatzer duct occluder. Iran J Pediatr. 2013 Aug;23(4):411-6.[pubmed]
Copyright (c) 2019 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


