A prospective study on the pharmacotherapy of bronchial asthma in pediatric patients at a tertiary care hospital; emphasis on adverse drug reactions
Abstract
Background: Paediatric asthma is one of the most common chronic illnesses in childhood and affects the quality of life in children. Anti-asthmatic drugs used in children may result in a beneficial and adverse drug reaction (ADR) and could contribute significantly to morbidity and mortality. Several studies report about the safety and efficacy of asthma medications in adults but the information in children is limited. Hence, the current study was aimed to investigate about safety and efficacy of anti-asthmatic drugs in children.
Material and Methods: A prospective, observational, non-interventional study of children who presented between January 2018 and December 2019 to the Department of Paediatric of Noor Hospital. Pediatric patients of bronchial asthma (both acute and chronic cases) of either gender within the age limit of 1-13 years who attended the outpatient department (OPD) as well as the inpatient department (IPD) were included in the study. Patients who are <1 and >13 years, patients with other co-morbid conditions like TB, Diabetes/renal failure or any other systemic disorders, or who were immunocompromised were excluded.
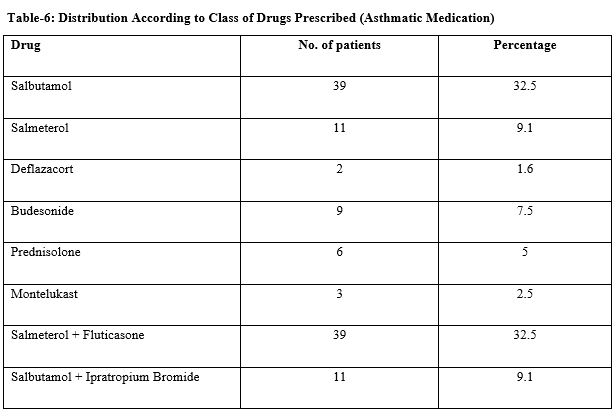
Results: Out of 120 patients, most of the pediatric patients suffering from asthma 42% were found in the age group of 5-8 years followed by (39%) 1-4 years and the last one is 9-13 years (19%). Demographic analysis of data revealed that there were 61.6% male and 38.4% female in the study. Out of 120 Paediatric asthma patients, 34.1% were suffering from mild persistent and the remaining 59.1% were patients of moderate persistent and 6.6% are least one of severe asthma. The percentages of the patients who were 58.4 % treated with a single anti-asthmatic drug (monotherapy) excluding other concomitant medications used together. 41.6 % of children were treated with anti-asthmatic drug combinations. The overall utilization of Anti-asthmatic drugs among pediatric asthma patients was found to be short-acting β2 Agonists (32.5%) long-acting β2agonist (LABA) (9.1%), steroids (14.1%) and leukotriene modifiers (2.5%). The most commonly reported ADRs were 2.5% of headache, 1.6% of palpitation, dryness of mouth, sore throat, anorexia, and 0.8% of oral candidiasis nausea/vomiting.
Conclusion: It has been concluded that a study may be more meaningful to further improve the prescribing as well as dispensing practices of the pharmacist through the successful implementation of interventional programs in health centers.
Downloads
References
Shaji J, Lodha S. Management of Asthma: A Review. Indian J Hosp Pharmacy. 2008;45:88-100.
American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatr. 2006;118(4):1774–1793. doi: https://doi.org/10.1542/peds.2006-2223.
Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007;356(20):2040-2052. doi: https://doi.org/10.1056/nejmoa063861.
Mishra N, Rao KVR, Padhi Sk. Asthma education for better compliance in disease management. Indian J Allergy Asthma Immunol. 2005;19:25-28.
Golshan M, Amra B, Zadeh ZM. Prevalence of asthma in high school adolescents of Isfahan. Med J Iran Hosp. 2001;4:35-40.
Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008;32(3):545-554. doi: https://doi.org/10.1183/09031936.00155307.
Anil K, Tiwari HK, Kulkarni SK. Drug utilization Assessment in Asthma Therapy through prescription Monitoring. Indian J Hosp Pharmacy. 2004;2:70-72.
Ip M, Lam K, Yam L, Kung A, Ng M. Decreased bone mineral density in premenopausal asthma patients receiving long-term inhaled steroids. Chest. 1994;105(6):1722-1727. https://doi.org/10.1378/chest.105.6.1722
Global Initiative for Asthma. Global strategy for asthma management and prevention: NHLBI/WHO workshop report. Bethesda, MD: National heart, lung, and blood institute, 2006. Aust Prescrib. 2001;24:29-31. Available at https://www.who.int/respiratory/asthma/GINA_WR_2006_copyright%5B1%5D.pdf.
Ungar WJ, Coyte PC. Prospective study of the patient level cost of asthma care in children. Pediatr Pulmonol. 2001;32(2):101-108.
Johnson CE. Aerosol corticosteroids for the treatment of asthma. Drug Intell Clin Pharm. 1987;21(10):784-790. doi: https://doi.org/10.1177/106002808702101002.
Taburet AM Schmit B. Pharmacokinetic optimization of asthma treatment. Clin Pharmacokinet 1994;26(5):396-418. doi: https://doi.org/10.2165/00003088-199426050-00006.
Arumugam V, Preeti K, Vijay J, Awanish P, Poonam T. Drug Utilization Assessment in Asthma Therapy through Prescription Monitoring at Dehradun Hospitals; Indian J Allergy Asthma Immunol. 2008;22(1):15-18.
Patel PD, Patel RK, Patel NJ; Analysis of Prescription Pattern and Drug Utilization in Asthma Therapy. Int Res J Pharm. 2012; 257-260.
RD Shimpi, PS Salunkhe, SR Bavaskar, GP Laddha, A Kalam A Khalik Patel; Drug utilization evaluation and prescription monitoring in asthmatic patients; Int J Pharm Biol Sci. 2012;2(1):117-122.
Sen EF, Verhamme KM, Neubert A, Hsia Y, Murray M, Felisi M, et al. Assessment of pediatric asthma drug use in three European countries; a TEDDY study. Europe J Pediatr. 2011;170(1):81-92. doi: https://doi.org/10.1007/s00431-010-1275-7.
Elkout H, Helms PJ, Simpson CR, McLay JS. Changes in primary care prescribing patterns for paediatric asthma: a prescribing database analysis. Arch Dis Childhood. 2012;97(6):521-525. doi: https://doi.org/10.1136/adc.2010.206268.
Greenstone II, Chroinin MN, Lasserson TJ, Ducharme F. Combination of inhaled long‐acting beta2‐agonists and inhaled steroids versus higher dose of inhaled steroids in children and adults with persistent asthma. Cochrane database of systematic reviews. 2005(4): CD005533. doi: https://doi.org/10.1002/14651858.cd005533.
Ducharme FM, Chroinin MN, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews. 2010(4):CD005533. doi: https://doi.org/10.1002/14651858.cd005533.pub2.
Thamby SA, Juling P, Xin BTW, N.C. Jing, Retrospective studies on drug utilization patterns of asthmatics in a Government hospital in Kedah, Malaysia, Int. Curr Pharm J. 2012;1(11):353–360. doi: http://dx.doi.org/10.3329/icpj.v1i11.12060.
Rao C., Ramakrishnan K.G., Somashekar A. R. Patterns of health care for children with asthma: A qualitative study. J Pediatr Res.2017;4(7):446-452. doi: https://doi.org/10.17511/ijpr.2017.07.03.
NAEPP Expert Panel Report: guidelines for the diagnosis and management of asthma-updates on selected topics 2002. Bethesda, MD: National heart, lung, and blood institute, 2002. (NIH Publication No. 02-5075). Available at https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma.
Barben J, Kuehni CE, Trachsel D, Hammer J, Swiss Paediatric Respiratory Research G. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice? Thorax. 2008;63(12):1103-1109. doi: https://doi.org/10.1136/thx.2007.094706.
M.F. Akram, M. Nasiruddin, Z. Ahmad, R.A. Khan, Doxofylline and theophylline: a comparative clinical study, J Clin Diagn Res. 2012;6 (10):1681-1684. doi: https://dx.doi.org/10.7860%2FJCDR%2F2012%2F4697.2643.
S.M. Margay, S. Farhat, S. Kaur, H.A. Teli, To study the efficacy and safety of doxophylline and theophylline in bronchial asthma, J Clin Diagn Res. 2015;9(4):5-8. doi: https://doi.org/10.7860/jcdr/2015/12438.5743.
K. Sayadeda, N.A. Ansari, Q.S. Ahmed, P. Upadhyay, S. Dey, A. Madhwar, Drug utilization study of antiasthmatic drugs in paediatric age group in a tertiary care teaching hospital, Bareilly, UP – India, Int. J. Univ. Pharm. Biosci. 2013;2(3):145-156.
Arellano FM, Arana A, Wentworth CE, Vidaurre CF, Chipps BE. Prescription patterns for asthma medications in children and adolescents with health care insurance in the United States. Pediatr Allergy Immunol. 2011;22(5):469-476. doi: https://doi.org/10.1111/j.1399-3038.2010.01121.x.
T. Rajathilagam, T. Sandozi, A.D. Nageshwari, P. Paramesh, R. Jamunarani, Drug utilization study in bronchial asthma in a tertiary care hospital, Int. J. Pharm. 2012;3(2):297-305.
Bygdell M, Brunlöf G, Wallerstedt SM, Kindblom JM. Psychiatric adverse drug reactions reported during a 10‐year period in the Swedish pediatric population. Pharmacoepidemiol Drug Saf. 2012;21(1):79-86. doi: https://doi.org/10.1002/pds.2265.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clinical Pharmacology & Therapeutics. 1981;30(2):239-245. doi: https://doi.org/10.1038/clpt.1981.154.
Gupta R, Fonacier LS. Adverse effects of nonsystemic steroids (inhaled, intranasal, and cutaneous): a review of the literature and suggested monitoring tool. Curr Allergy Asthma Rep. 2016;16(6):44. doi: https://doi.org/10.1007/s11882-016-0620-y.
Copyright (c) 2020 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


