Neuroprotective dose and safety profile of magnesium sulphate therapy in term neonates with perinatal asphyxia
Abstract
Introduction: Perinatal asphyxia has become a major public health problem accounting for 9% of total under-5 mortality. It can lead to serious neuro-motor sequelae in survivors. Newer neuroprotective strategies for management of perinatal asphyxia have gained momentum in recent era.
Background: To determine the effective dosage regimen and safety profile of intravenous magnesium sulphate as a neuroprotective agent for perinatal asphyxia in term neonates.
Materials and methods: 60 term asphyxiated neonates admitted in NICU of a tertiary care teaching hospital were evaluated. Babies were treated as per the standard treatment protocol for perinatal asphyxia. The infants received 250 mg/kg per dose intravenous magnesium over 1 hour within 6 hrs of birth, with 2 additional doses repeated at intervals of 24 hours. The heart rate, respiratory rate, blood pressure and oxygen saturation were monitored continuously. A baseline serum magnesium level was measured soon after delivery and two more serum magnesium levels at 24 hr and at 48 hr was measured.
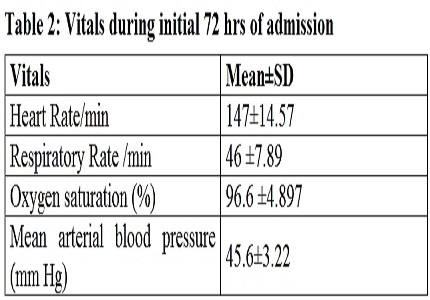
Results: The pre intervention baseline serum magnesium level was 1.52 (±0.302) meq/L. The serum magnesium level increased from the baseline level of 1.52 (±0.302) meq/L to 2.63 (±0.558) meq/L at 24 hour and 2.72 (±0.495) meq/L at 48 hour. The neuroprotective range of serum magnesium is 2.4 – 5 Meq/L. This dosage regimen will ensure plasma concentration of magnesium in the neuroprotective range for 48 hours. All physiologic variables remained unchanged including heart rate, respiratory rate, blood pressure and oxygen saturation during intervention.
Conclusion: Intravenous infusion of 3 doses of magnesium 250 mg/kg/dose in first 48 hrs after birth will ensure serum magnesium level in the neuroprotective range. This therapeutic dosage regimen has been found to have the best margin of safety profile in term asphyxiated infants.
Downloads
References
2. Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006 Mar;117(3 Pt 2):S28-33.
3. Gathwala G. Neuronal protection with magnesium. Indian J Pediatr. 2001 May;68(5):417-9.
4. Chahal H, D'Souza S, Barson A, Slater P. Modulation by magnesium of N-methyl-D-aspartate receptors in developing human brain. Archives of Disease in Childhood - Fetal and Neonatal Edition. 1998; 78(2):F116-F120.
5. Antonucci R, Porcella A, Pilloni MD. Perinatal asphyxia in the term newborn. J Pediatr Neonat Individual Med. 2014; 3(2):e030269. doi: 10.7363/030269.
6. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976 Oct;33(10):696-705.
7. Sinha SK, Singh J. Newer concepts and approaches to neonatal brain asphyxia. Indian J Pediatr. 1998 Jan-Feb;65(1):55-62.
8. Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171-82.
9. Haddad JJ. N-methyl-D-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: A revolving neurochemical axis for therapeutic intervention? Progress in Neurobiology. 2005; 77:252–282.
10. Levene M, Blennow M, Whitelaw A, Hankø E, Fellman V, Hartley R. Acute effects of two different doses of magnesium sulphate in infants with birth asphyxia. Arch Dis Child Fetal Neonatal Ed. 1995 Nov;73(3):F174-7.
11. Bhat MA, Charoo BA, Bhat JI, Ahmad SM, Ali SW, Mufti MU. Magnesium sulfate in severe perinatal asphyxia: a randomized, placebo-controlled trial. Pediatrics. 2009 May;123(5):e764-9. doi: 10.1542/peds.2007-3642. Epub 2009 Apr 6.
12. Ichiba H, Tamai H, Negishi H, Ueda T, Kim T, Sumida Y et al. Randomized controlled trial of magnesium sulfate infusion for severe birth asphyxia. Pediatrics International. 2002; 44(5):505-509.
13. Ichiba H, Yokoi T, Tamai H, Ueda T, Kim TJ, Yamano T. Neurodevelopmental outcome of infants with birth asphyxia treated with magnesium sulfate. Pediatr Int. 2006 Feb;48(1):70-5.
14. Gathwala G, Khera A, Singh I. Magnesium therapy in birth asphyxia. Indian J Pediatr. 2006 Mar;73(3):209-12.
Copyright (c) 2016 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


