Wilms Tumor Treatment Outcome in Bangladesh Shishu Hospital & Institute, Dhaka, Bangladesh
Abstract
Background: Wilms tumor is the commonest renal malignancy in childhood. Survival in high-income countries is approximately 90%, whereas in low-income countries, it is less than 60%. This study assessed treatment outcomes of patients with Wilms tumor at Bangladesh Shishu Hospital & Institute.
Patients and Methods: We conducted a prospective study in all children diagnosed with Wilms tumor between July 2020 and July 2024. Total 30 patients of Wilms tumor were enrolled in our study, Data were collected such as treatment outcomes and various socio demographic and clinical characteristics and recorded in sheet.
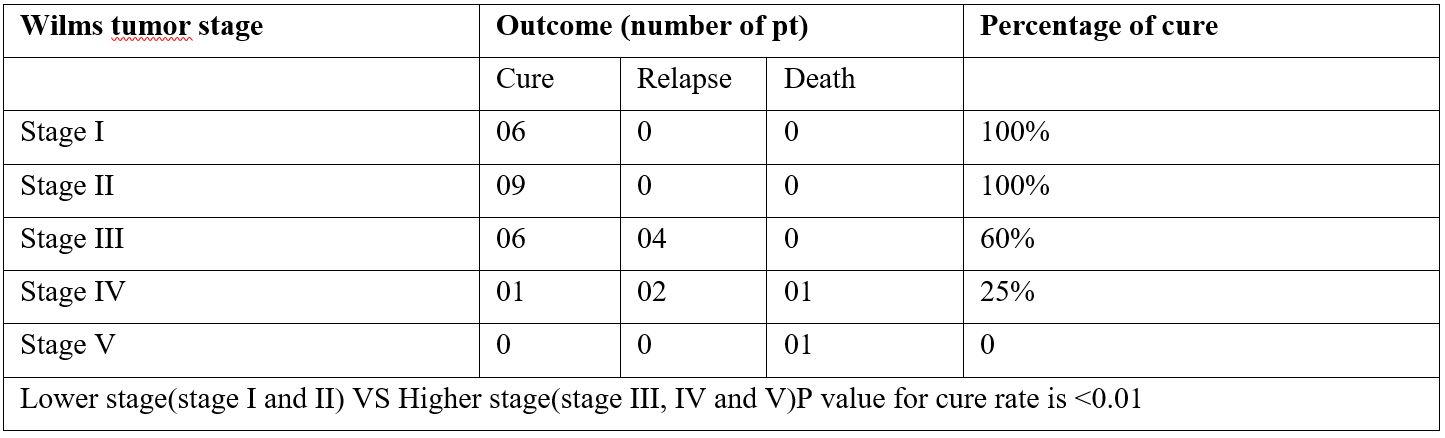
Results: Of the 30 patients with Wilms tumor, 73.33% had event-free survival, 20% had relapsed, 6.67% died. Patients presented as follows-stage I (20%), II (25%), III (35%), IV (15%), or V (5%). The most likely treatment outcome in patients with low-stage (I to II) disease was event-free survival (100%), whereas in those with high-stage (III,IV and V) disease, it was 46.67%. No deaths or instances of progressive or relapsed disease were recorded among patients with low-stage disease. Stage of disease significantly affected treatment outcomes (P =0 .01) and event-free survival estimates (P < .001). Age at diagnosis, sex, duration of symptoms did not statistically significantly influence treatment outcomes or event-free survival estimates.
Conclusion: Survival of patients with Wilms tumor in Bangladesh is lower compared with that in high-income countries. Treatment abandonment is the most common cause of treatment failure. Stage of disease at diagnosis statistically significantly affects treatment outcomes and survival.
Downloads
References
2.Rabeh W, Akel S, Eid T, et al: Wilms tumor: Successes and challenges in management outside of cooperative clinical trials. Hematol Oncol Stem Cell Ther 9:20-25, 2016.
3.Wilde JC, Lameris W, van Hasselt EH, et al: Challenges and outcome of Wilms’ tumour management in a resource-constrained setting. Afr J Paediatr Surg 7:159-162, 2010.
4.Dome JS, Perlman EJ, Graf N: Risk stratification for wilms tumor: Current approach and future directions. Am Soc Clin Oncol Educ Book 215-223, 2014.
5.Dome JS, Graf N, Geller JI, et al: Advances in Wilms tumor treatment and biology: Progress through international collaboration. J Clin Oncol 33:2999-3007, 2015.
6. Oostveen RM, Pritchard-Jones K. Pharmacotherapeutic Management of Wilms Tumor: An Update. Paediatr Drugs. 2019 Feb.
7. Nakata, K., Colombet, M., Stiller, C. A., Pritchard-Jones, K. & Steliarova-Foucher, E. Incidence of childhood renal tumours: an international population-based study. Int. J. Cancer 147, 3313–3327 (2020).
8. Caldwell BT, Wilcox DT, Cost NG. Current Management for Pediatric Urologic Oncology. Adv Pediatr. 2017 Aug;64(1):191-223.
9. Han Q, Li K, Dong K, Xiao X, Yao W, Liu G. Clinical features, treatment, and outcomes of bilateral Wilms' tumor: A systematic review and meta-analysis. J Pediatr Surg. 2018 Dec;53(12):2465-2469.
10.Aldrink JH, Heaton TE, Dasgupta R, et al. Update on Wilms tumor. J Pediatr Surg. 2019;54(3):390-397.
11. Tenge CN, Were PA, Aluoch LH, et al: Management and outcomes of patients with nephroblastoma at the Moi Teaching and Referral Hospital Eldoret, Kenya. East Afr Med J 89:121-127, 2012.
12. Lauren N. Parsons, MD,1Elizabeth A. Mullen et al:Outcome Analysis of Stage 1 Epithelial Predominant Favorable Histology Wilms tumors.Eur JCancer. 2020 Jun 15; 126(12): 2866–2871.
13. Grundy PE, Breslow NE, Li S et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 2005; 23:7312–7321.
14. Dome JS, Cotton CA, Perlman EJ et al. Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol 2006; 24:2352–2358.
15. Pritchard-Jones K, Moroz V, Vujanic G, et al: Treatment and outcome of Wilms’ tumour patients: An analysis of all cases registered in the UKW3 trial. Ann Oncol 23:2457-2463, 2012.
16.Yuo W, Li K, Xiao X, et al: Outcomes of Wilms’ tumor in eastern China: 10 years of experience at a single center. J Invest Surg 25:181-185, 2012.
17.Paintsil V, David H, Kambugu J, et al: The Collaborative Wilms Tumour Africa Project: Baseline evaluation of Wilms tumour treatment and outcome in eight institutes in sub-Saharan Africa. Eur J Cancer 51:84-91, 2015.
18.Israels T, Borgstein E, Pidini D, et al: Management of children with a Wilms tumor in Malawi, sub-Saharan Africa. J Pediatr Hematol Oncol 34:606-610, 2012.
19.Moreira C, Nachef MN, Ziamati S, et al: Treatment of nephroblastoma in Africa: Results of the first French African pediatric oncology group (GFAOP) study. Pediatr Blood Cancer 58:37-42, 2012.
20.Stones DK, Hadley GP, Wainwright RD, et al: The impact of ethnicity on Wilms tumor: Characteristics and outcome of a South African cohort.
21.Israels T, Challinor J, Howard S, et al: Treating children with cancer worldwide: Challenges and interventions. Pediatrics 136:607-610, 2015.
22.Njuguna F, Martijn H, Langat S, et al: Factors influencing time to diagnosis and treatment among pediatric oncology patients in Kenya. Pediatr Hematol Oncol 33:186-199, 2016.
Copyright (c) 2024 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


