Type II hypersensitivity and trimethoprim-sulfamethoxazole
Abstract
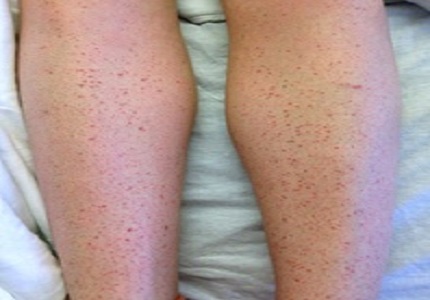
Trimethoprim-sulfamethoxazole (Septra) is a widely used antibiotic world-wide.The clinical use has been increasing in the pediatric population[1]. Septra has been associated with a broad array of drug associated reactions including gastrointestinal complaints, cutaneous reactions including Stevens-Johnson syndrome and toxic epidermal necrolysisand cytopenias including immune mediated thrombocytopenia[2]. Adverse reactions occur in 6-8% of patients. In the pediatric patient hospitalized for an adverse drug reaction priorexposure to Septra is found in 75% of patients [3].In the case presented we describe a cutaneous reaction to Septra clinically consistent with a Type2 hypersensitivity reaction with associated pancytopenia. Idiosyncratic reactions such as Type 2 hypersensitivity have rarely been reported with Septra exposure.These adverse drug reactions have infrequently been reported to be fatal [4].With the increasing use of Septra for the management of community acquired methicillin resistant Staphylococcus aureus of skin and soft tissue infections [5] clinicians will need to recognize this clinical complication.
Downloads
References
2. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007; 357(6):580–587. [PubMed]
3. Goldman J, Jackson MA, Herigon JC, Hersh AL, Shapiro DJ, Leeder JS. Trends in Adverse Reactions to Trimethoprim-Sulfamethoxazole.Pediatrics. 2013; 131(1): e103-e108.doi: 10.1542/peds.2012-1619. [PubMed]
4. Kocak Z, Hatipoglu CA, Ertem G, Kinikli S, Tufan A, Irmak H,, et al.. Trimethoprim-sulfamethoxazole induced rash and fatal hematologic disorders. JInfect. 2006; 52(2):e49-52. [PubMed]
5. Lee MC, Rios AM, Aten MF, Mejias A, Cavuoti D, McCracken GH, Jr, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr InfectDis J. 2004; 23(2):123–127. [PubMed]
6. Curtis BR. Drug-induced immune neutropenia/agranulocytosis.Immunohematology. 2014;30(2):95-101. [PubMed]
7. SchopfE. Skin reactions to co-trimoxazole. Infection 1987; 15 Suppl 5: S254-258. [PubMed]
8. Pichler WJ. Drug allergy: classification and clinical features. In: UpToDate,Feldweg AM (Ed), Waltham, MA, 2013.http://www.uptodate.com.(Accessed on April 26, 2014.)
9. MainraRR, Card SE.Trimethoprim–sulfamethoxazole-associated hepatotoxicity—part of a hypersensitivity syndrome. Canadian Journal of Clinical Pharmacology.2003; 10 (4):175-178
Copyright (c) 2016 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


