Minocycline trial in japanese encephalitis: a double blind, randomized placebo study
Abstract
Objective: To evaluate the effect of minocycline in Japanese Encephalitis (JE ) patient on reduction in mortality, neurological deficit and behavioral outcome.
Design: Double blind randomized placebo control trial.
Setting: Tertiary level hospital.
Inclusion criteria: Only JE patients who were IgM positive for JE Virus (JEV) in cerebrospinal fluid and/or serum were included.
Exclusion criteria: Those clinically suspected JE patients who were IgM negative for JE Virus (JEV) in cerebrospinal fluid and serum were excluded. Patients were selected randomly.
Intervention: A total of 44 patients of JE of the age range between 1-13yr were divided equally in two groups A and B. Group A received drug under study, that is minocycline, and Group B received placebo. Outcome measures: The present study was designed to: compare the clinical course during hospitalization and after discharge in terms of duration of symptoms, death and disability at the time of discharge, outcome of the intervention on clinical recovery, behavioral problems and neurological deficit after 12 month of follow up.
Results: Duration of fever, unconsciousness and total duration of hospital stay were significantly reduced in Group A (minocycline) patients.
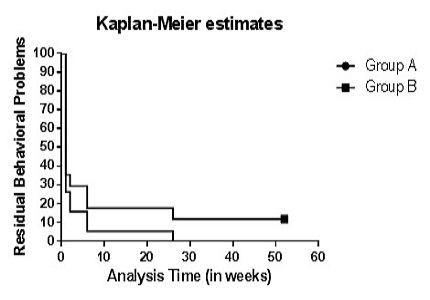
Conclusion: Minocycline has significant beneficial effect in JE patients in duration of major symptoms and duration of hospital stay. However, the mortality rate and prevalence of neurological deficits and behavioral problems on a 12 months follow up remain unchanged.
Downloads
References
2. Parida M, Dash PK, Tripathi NK, Ambuj, Sannarangalah S, Saxena P, et al. Japanese encephalitis outbreak, India. Emerg Infect Dis. 2006; 12: 1427–30. [PubMed]
3. Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob Agents Chemother. 2007 Sep;51(9):3367-70. Epub 2007 Jun 18. [PubMed]
4. Stancek d, Vilcek j, The role of interferon in tick-borne encephalitis virus-infected l cells. I. Acute infection. Acta virol. 1965; 9:1-8.
5. Dutta K, Ghosh D and Basu A. Curcumin Protects Neuronal Cells from Japanese Encephalitis Virus-Mediated Cell Death and also Inhibits Infective Viral Particle Formation by Dysregulation of Ubiquitin–Proteasome System. Journal of neuroimmune pharmacology 2009; 4(3): 328- 337.
6. Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem. 2008; 105 (5):1582-95.
7. Anantpadma M and Vrati S. SiRNA-mediated suppression of Japanese encephalitis virus replication in cultured cells and mice.J. Antimicrob. Chemother. 2011; 67 (2):444-451. [PubMed]
8. Kumar R, Tripathi P, Baranwal M, Singh S, Tripathi S, Banerjee G. Randomized, controlled trial of oral ribavirin for Japanese encephalitis in children in Uttar Pradesh, India. Clinical Infectious Diseases. 2009; 48: 400-4.
9. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006; 54:258–65.
10. Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007; 6: 1045-53. [PubMed]
11. Bonelli RM, Hödl AK, Hofmann P, Kapfhammer HP. Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol. 2004; 19(6):337-342. [PubMed]
12. Zink MC et al. "Neuroprotective and anti-human immunodeficiency virus activity of minocycline". JAMA . 2005; 293 (16): 2003–11. [PubMed]
13. Zabad RK, Metz LM, Todoruk TR, et al. The clinical response to minocycline in multiple sclerosis is accompanied by beneficial immune changes: a pilot study. Mult Scler. 2007; 13 (4):517-526.
14. Zhang Y, Metz LM, Yong VW, et al. Pilot study of minocycline in relapsing remitting multiple sclerosis. Can J Neurol Sci. 2008;35(2):185-191. [PubMed]
15. Lemaitre M, Guetard D, Henin Y, Montagnier L, Zerial A. Protective activity of tetracycline analogs against the cytopathic effect of the human immunodeficiency viruses in CEM cells. Res Virol. 1990;141:5–16.
16. Dutta K, Kumawat KL, Nazmi A, Mishra MK, Basu A. Minocycline differentially modulates viral infection and persistence in an experimental model of Japanese encephalitis. J Neuroimmune Pharmacol. 2010; 5(4): 553-65.
17. Michaelis M, Kleinschmidt MC, Doerr HW, Cinatl J Jr. Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. . J. Antimicrob Chemother. 2007; 60(5): 981-6. [PubMed]
18. Sapkal GN, Wairagkar NS, Ayachit VM, Bondre VP, Gore MM. Detection and isolation of Japanese encephalitis virus from blood clots collected during the acute phase of infection. Am J Trop Med Hyg. 2007; 77: 1139–45. [PubMed]
19. Lewthwaite P, Begum A, Ooi MH, etal. Disability after encephalitis: development and validation of a new outcome score. Bulletin of the World Health Organization 2010; 88:584-592. doi: 10.2471/BLT.09.071357. [PubMed]
20. Thomas M. Achenbach, Thomas M. Ruffle. The Child Behavior Checklist and Related Forms for Assessing Behavioral/Emotional Problems and Competencies. Pediatrics in Review Aug 2000, 21 (8) 255; doi: 10.1542/pir.21-8-255. [PubMed]
21. Labro MT. Immunomodulatory effects of antimicrobial agents. Part I: antibacterial and antiviral agents. Expert Rev Anti Infect Ther. 2012; 10(3):319-40. [PubMed]
22. Chong CR, Sullivan DJ Jr. New uses for old drugs. Nature. 2007; 448: 645-6. [PubMed]
23. Dutta K & Basu A. Use of minocycline in viral infections. Indian J Med Res. 2011; 133: 467-470. [PubMed]
24. Mishra MK, Ghosh D, Duseja R, Basu A. Antioxidant potential of minocycline in Japanese Encephalitis virus infection in murine neuroblastoma cells: correlation with membrane fluidity and cell death. neurochem Int. 2009; 54 : 464-70.
25. Mishra MK, Dutta K, Saheb SK, Basu A. Understanding the molecular mechanism of blood-brain barrier damage in an experimental model of Japanese encephalitis: correlation with minocycline administration as a therapeutic agent. Neurochem Int. 2009; 55: 717-23.
26. Jackson AC. Is minocycline useful for therapy of acute viral encephalitis? Antiviral Res. 2012; 95(3):242-4. [PubMed]
27. Yik et al. Neuroprotective effects of minocycline on double-stranded RNA-induced neurotoxicity in cultured cortical neurons. Hong Kong Med J. 2012; 2:42-44. [PubMed]
28. Sarkari NB,; Thacker AK,; Barthwal SP,; et al . Japanese encephalitis (JE) part II: 14 years' follow-up of survivors. J Neurol .2012; 259:58–69.
Copyright (c) 2016 Author (s). Published by Siddharth Health Research and Social Welfare Society

This work is licensed under a Creative Commons Attribution 4.0 International License.


 OAI - Open Archives Initiative
OAI - Open Archives Initiative


